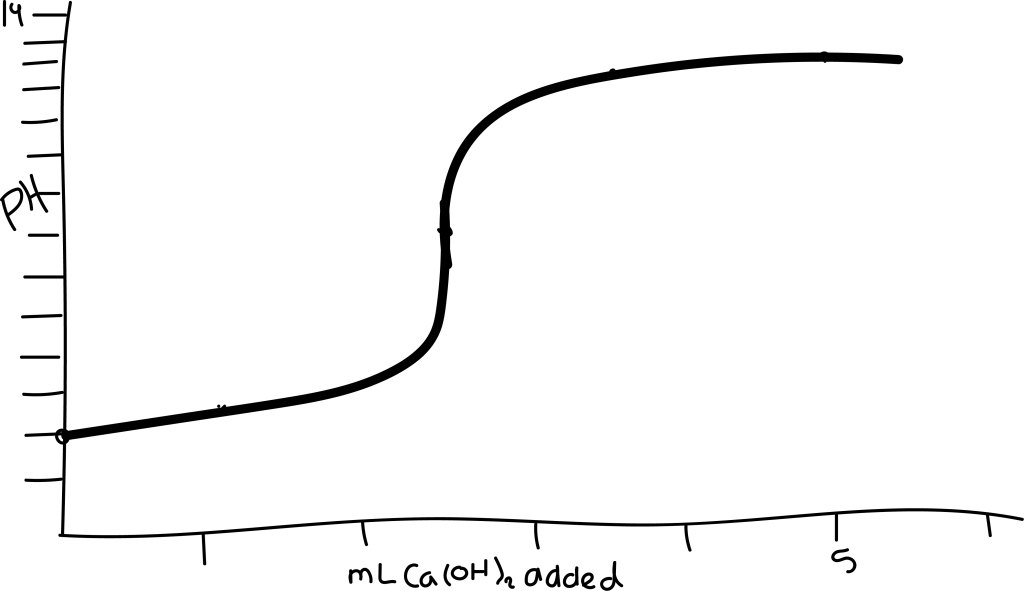
(a) Sketch the titration curve for the titration of 5.00 mL 0.010 M HCl (aq) with 0.010 M Ca(OH)2 (aq) showing the pH of the initial and final solutions and the pH at the stoichiometric point. What volume of titrant has been added at (b) the stoichiometric point; (c) the halfway point of the titration?
Atkins, P., & Jones, L. (2010). Chemical Principles: The Quest for Insight with Unique International Edition Problem Sets, Fifth Edition. New York, NY: W. H. Freeman and Company.
Initial pH of 5.00 mL 0.010 M HCl (aq)
Strong acids experience 100% ionization so the concentration of hydrochloric acid will have a one-to-one ratio to its products, hydrogen ion (proton) and chloride ion.
HCl (aq) –> H+ (aq) + Cl- (aq)
[HCl] = [H+] therefore, 0.010 M HCl = 0.010 M H+
pH = -log([H+]) therefore, pH= -log(0.010 M)
The initial pH of 5.00 mL 0.010 M HCl (aq) is equal to 2. The titration curve will have an x-axis labeled mL of Ca(OH)2 added and a y-axis labeled pH. The first coordinate will be (0.00 mL base added, pH 2).
Volume of 0.010 M Ca(OH)2 at the Stoichiometric Point with 5.00 mL 0.010 HCl
For any titration, moles of acid is equal to moles of base at the stoichiometric point.
| 5.00 mL HCl | 1 L HCl | 0.010 mol HCl | 1 mol H+ | 1 mol OH- | 1 mol Ca(OH)2 | 1 L Ca(OH)2 | 1000 mL Ca(OH)2 |
| 1 | 1000 mL HCl | 1 L HCl | 1 mol HCl | 1 mol H+ | 2 mol OH- | 0.010 mol Ca(OH)2 | 1 L Ca(OH)2 |
Using the conversion table above from mL HCl to mL Ca(OH)2, 2.5 mL of 0.010 M Ca(OH)2 is needed to reach the stoichiometric point wheres moles of acid will equal moles of base.
An alternative method can be used to solve for the volume of calcium hydroxide at the stoichiometric point with the equation MaVa = MbVb, where concentration of acid times volume of acid is equal to concentration of base times volume of base.
It is important to note that calcium hydroxide contains 2 moles of hydroxide ions, therefore the molarity of base will be 0.02 M to use in the equation:
(0.01 M acid)(5.00 mL acid) = (0.02 M base)(Vb), where Vb = 2.5 mL base
Stoichiometric pH of 5.00 mL 0.010 M HCl (aq) and 2.5 mL 0.010 M Ca(OH)2
Strong-acid-strong-base neutralization reactions will have a pH equal to 7 at the stoichiometric point. All moles of hydrogen ion have reacted with all moles of hydroxide ions to give the net ionic equation:
H+(aq) + OH-(aq) –> H2O(l)
| H+(aq | OH-(aq) | –> | H2O(l) | |
| initial moles | 5 E-5 mol | 0 | x | 0 |
| change: added OH- | -5E-5 mol | all moles react with H+ | x | +5E-5 mol |
| final moles | 0 mol | 0 mol | x | 5E-5 mol |
Since the only remaining compound in solution is H2O(l), The autoionization constant of water, Kw, can help to confirm the pH of the solution at the stoichiometric point:
Kw=1E-14=[OH-][H+], where x=[OH-]=[H+]
1E-14 = x^2, where x= 1E-7, therefore [H+]= 1E-7, and pH= -log(1E-7)
The pH of the neutralization reaction at the stoichiometric point will be equal to 7, and is a neutral solution.
The coordinate for the stoichiometric point on the graph will be (2.5 mL base added, pH 7).
Volume of 0.010 M Ca(OH)2 added at the halfway point of the titration of 5.00 mL 0.010 M HCl
Since we know 2.5 mL of calcium hydroxide is needed to reach the stoichiometric point, this volume of base is halved to reach the halfway point of the titration, therefore 1.25 mL of 0.010 M Ca(OH)2 is added at the halfway point of titration of 5.00 mL 0.010 M HCl.
The halfway point of the titration is also where half the moles of acid has reacted with base and half the moles of the acid still remains unreacted in the solution.
H+(aq) + OH-(aq) –> H2O(l)
0.010 M Ca(OH)2= 0.020 M OH-, therefore, 0.020 mol OH- is added per every L of solution and 1.25E-3 L solution will add 2.5E-5 mol OH- to the reaction at the halfway point of titration:
| H+(aq | OH-(aq) | –> | H2O(l) | |
| initial moles | 5 E-5 mol | 0 | x | 0 |
| change: added OH- | -2.5E-5 mol | all moles react with H+ | x | +2.5E-5 mol |
| final moles | 2.5E-5 mol | 0 mol | x | 2.5E-5 mol |
The pH of the solution at the halfway point of titration will depend solely on the leftover amount of hydrogen ions in solution and the total volume present after the addition of 1.25 mL base.
The concentration of hydrogen ions at the halfway point is equal to 2.5E-5 mol H+ divided by 6.25E-3L total volume of solution:
[H+] = 2.5E-5 mol/6.25E-3 L = 0.004 M H+, therefore pH = – log(0.004M) = 2.40
The coordinate for halfway point of titration to sketch on the graph will be (1.25 mL base added, pH 2.40)
pH of the Final Solution for the Titration of 5.00 mL 0.010 M HCl with 0.010 M Ca(OH)2
To complete the sketch of the titration graph, an accurate representation of the pH after the stoichiometric point needs to be shown. Once we have reached the stoichiometric point for a strong-acid-strong-base neutralization reaction, the pH is calculated based solely on the concentration of hydroxide ions added in excess. These hydroxide ions are added “in excess” because all moles of acid have reacted. Any volume of base that is greater than the amount needed to reach the stoichiometric point is effective for this calculation: 3.75 mL and 5 mL of Ca(OH)2 will be used.
5E-5 moles OH- ions will react with moles of H+ ions to reach the stoichiometric point.
3.75E-3 L base with a concentration of 0.020 M OH- ions will yield a total of 7.5E-5 moles OH-. There will be 2.5E-5 moles OH- in excess with a total solution volume of 8.75 mL. The concentration of hydroxide ions will be equal to 2.5E-5 mol/ 8.75E-3 L= 0.0025 M OH-.
The pOH of the solution will be equal to -log(0.0025 M)= 2.60 , therefore the pH will be equal to 14-pOH = 11.40 when 3.75 mL of base is added.
On the sketch of the titration graph, a coordinate will be (3.75 mL base added, pH 11.40).
5E-3 L base with a concentration of 0.020 M OH- ions will yield a total of 1E-4 moles OH-. There will be 5E-5 moles OH- in excess with a total solution volume of 10 mL. The concentration of hydroxide ions will be equal to 5E-5 mol/10E-3 L = 0.005 M OH-.
The pOH of the solution will be equal to -log(0.005 M) = 2.30, therefore the pH will be equal to 14-pOH = 11.70 when 5.00 mL of base is added.
On the sketch of the titration graph, a coordinate will be (5.00 mL base added, pH 11.70).
Sketch a Curve of the Titration of 5.00 mL 0.010 M HCl with 0.010 M Ca(OH)2
| mL base added | pH |
| 0 | 2 |
| 1.25 | 2.4 |
| 2.5 | 7 |
| 3.75 | 11.4 |
| 5 | 11.7 |